
Again the atomic radius is different because of their ionic status of the atom.The atoms of different element, they must differ in electrons and protons, sometimes number of orbitals and so on.Here the radius of two ions are not equal and few other things need to be discussed before we calculate the ionic radius: Ionic radius is the radius calculated when an atom is bonded with another atom in a molecule by transferring electrons to make ionic bond. The metallic diameter is equal to the distance between two nuclei and the radius is just half of it. The metallic radius can also be calculated by measuring the distance between the two nuclei of the two atoms. The metallic radius is the radius calculated between two metal atoms bonded together in metal cluster. The diameter of an atom is equal to the distance between two nuclei and the atomic radius will be the half of it. When an atom (A) is covalently bonded with another homonuclear atom (A), the atomic radius of that atom can be calculated by measuring the distance between the two nucleus of those two atoms. Covalent radiusĬovalent radius is the radius calculated when an atom is bonded covalently with another atom of same element. There are different methods of calculating the radius by measuring the distance between two nucleus when an atom is bonded in a molecule. So, as the position of electron is not certain rather they are explained as electron cloud around the nucleus, it is hard to measure an atomic radius accurately.

Ways to measure the atomic radiusĪccording to the Heisenberg uncertainty principle, it is not possible to measure the momentum and the position of the electron simultaneously. Thus sometime different methods are used to measure the radius while they are bonded in a molecule.
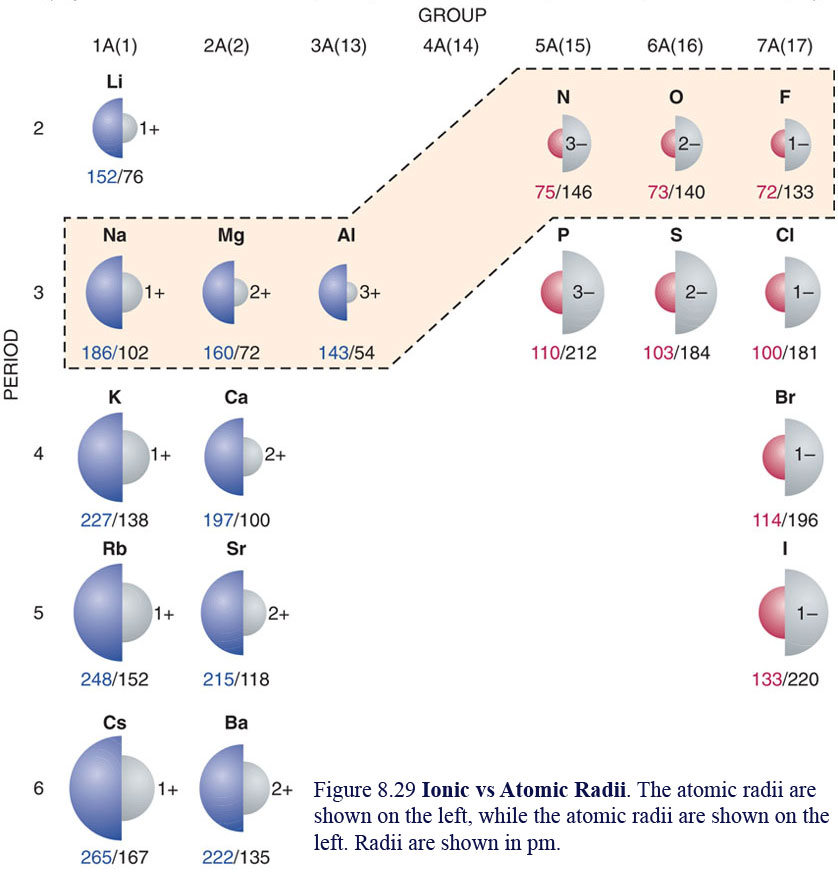
As there are no physical existence of orbital in atoms, it is difficult to measure the atomic radius. The atomic radius is the size of the atom, typically measured by the distance from the nucleus of the atom to the electron clouds around the nucleus.


 0 kommentar(er)
0 kommentar(er)
